-
AAHRPP Accreditation Procedures
Jan 18, 2022, 12:33 PM
Resources: For Accreditation - Procedures

Table of Contents
Issues Requiring Prompt Reporting and Annual Reports
EVENT REPORTING
An organization must report to AAHRPP as soon as possible but within 48 hours after the organization or any researcher (if the researcher is notified rather than the organization) becomes aware of:
- Any negative actions by a government oversight office, including, but not limited to:
- OHRP Determination Letters
- FDA Warning Letters
- FDA 483 Inspection Reports with official action indicated
- FDA Restrictions Placed on IRBs or researchers
- Compliance actions taken under non-US authorities related to human research protections
- Any litigation, arbitration, or settlements initiated related to human research protections
- Any press coverage (including but not limited to radio, TV, newspaper, online publications) of a negative nature regarding the organization’s HRPP
Failure to submit events requiring reporting within 48 hours as outlined above may result in an organization being placed into Accreditation-Pending, Reaccreditation-Pending, Probation, or Accreditation Revoked.
When an accredited organization or its HRPP has a substantive change, it should notify the AAHRPP office within 30 calendar days of the change. Examples of substantive changes include but are not limited to:
- Change in organization type or corporate structure.
- Change in ownership or control of the organization, including mergers or acquisitions.
If it is unclear to the organization whether a particular item is reportable to AAHRPP, the organization must contact AAHRPP for further advice.
When necessary, the Council will review information regarding the HRPP that is provided through the reporting mechanisms described above and will determine whether any action is indicated, such as a request for additional written information or a Limited Site Visit.
ANNUAL REPORTS
AAHRPP requires an accredited organization to submit an Annual Report one year after it receives Full Accreditation, Full Reaccreditation, or Qualified Accreditation. Annual Reports are required each year thereafter; however, an Annual Report is not required in the year in which the Application for Reaccreditation is submitted and the year in which the organization’s Application for Reaccreditation is reviewed by the Council on Accreditation (Council). However, if an organization is significantly delayed in submitting an Application for Accreditation or when the organization's complete application materials are not reviewed at the scheduled Council date, AAHRPP also may request an Annual Report. Failure to submit an Annual Report in any year in which it is due may result in an organization being placed into Accreditation-Pending, Reaccreditation-Pending, Probation, or Accreditation Revoked.
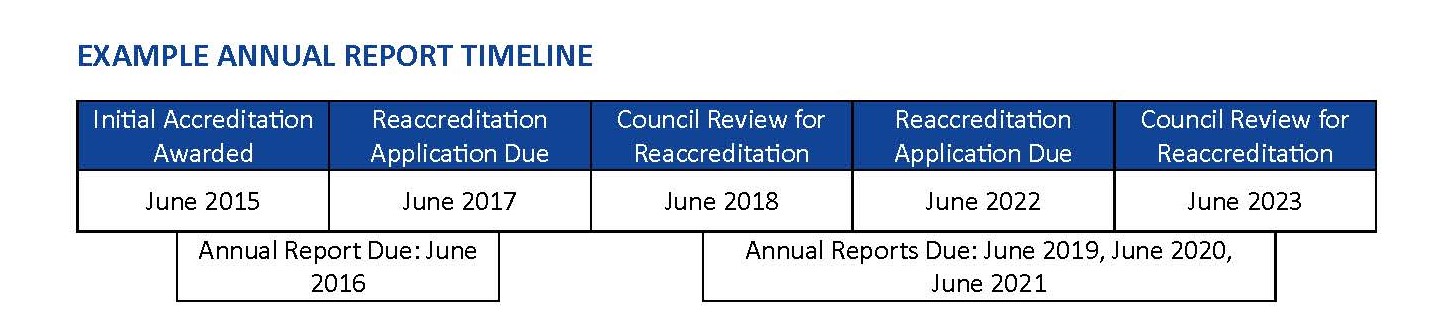
The purpose of the Annual Report is to keep AAHRPP current on the nature and size of an organization’s HRPP and human research portfolio as well as to notify AAHRPP of changes related to or that might affect the organization’s HRPP, including but not limited to:
- Organizational Changes, such as:
- Change in organization type.
- Change in corporate structure.
- Change in ownership or control of the organization, including mergers or acquisitions.
Note: For substantive changes such as these, an organization should notify the AAHRPP office within 30 calendar days of the change rather than waiting until providing the Annual Report. Questions in the Annual Report about events that have occurred at an organization serve as a backup mechanism to ensure AAHRPP receives information that could affect an organization’s accreditation.
- Changes in Resources, such as:
- Significant change (10% or more) in the balance of resources and active research protocols.
- Significant reduction (10% or more) in resources in the past 12 months and the impact on the HRPP, such as reduction in full-time equivalent (FTE) or dissolution of an IRB/EC, committee, or other function.
- Changes in Program Scope, such as:
- Addition of a new research program, including but not limited to a type of research not previously conducted or reviewed by the organization (such as planned emergency research, research involving children, or gene transfer research).
- Addition, removal, or modification of functions, committees, or IRBs/ECs.
- Changes in organizations that are entities of your HRPP.
- Changes in method of providing services, such as use of external IRBs/ECs or contracting for services from another organization.
- Catastrophic event that results in an interruption or discontinuance in a part of or the entire HRPP.

